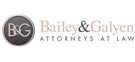Tequin
 Tequin, one of the brand names for the pharmaceutical product Gatifloxacin, was introduced to the U.S. market by Bristol-Myers Squibb in 1999. The drug, an antibiotic, was prescribed primarily for the treatment of respiratory tract infections.
Tequin, one of the brand names for the pharmaceutical product Gatifloxacin, was introduced to the U.S. market by Bristol-Myers Squibb in 1999. The drug, an antibiotic, was prescribed primarily for the treatment of respiratory tract infections.
In 2006, a report in the New England Journal of Medicine alleged that Tequin had been found to put users at an elevated risk for dysglycemia, a blood sugar metabolism disorder that may manifest as either hyperglycemia (high blood sugar) or hypoglycemia (low blood sugar). Initially, Bristol-Myers Squibb notified health care providers that product warnings would be enhanced. However, the company subsequently opted to terminate the manufacture of Tequin. The product is now available only as treatment for eye conditions, in the form of an eye drop.
If you experienced side effects after being prescribed Tequin, GetLegal has the tools and resources to help you find skilled legal representation to protect your rights. Visit our attorney directory, look for your geographic location and find the best lawyer for your situation.
Connect with Top-rated Attorneys Near You
Sponsored Advertisement
Defective Drugs & Medical Devices Topics
Accutane
Aciphex
Actonel
Actos
Adderall and Ritalin
Advair
Aldara
Alendronate
Alli
Ambien
Amiodarone
Anzemet
Aptivus
Aranesp
Arava
Aredia
Aricept
Avandia
Avastin
Avelox
Avodart
Avonex
Azathioprine
Benicar
Clozaril
Concerta
Coreg
Crestor
Cylert
Cymbalta
Cytotec
Darvocet
Darvon
Daypro
Essure Birth Control Medication
Gadolinium
Granuflo
Hydroxycut
Invokana
IVC Filters
Ketek
Levaquin
Mirapex
Neurontin
Onglyza
OxyContin
OxyContin Birth Defects
Paxil
Power Morcellators
Pradaxa
Propecia
Reglan
Risperdal
Surgical Mesh
Tequin
Trasylol
Viagra
Xarelto
Xolair
Zelnorm
Zoloft
Exactech Hip and Knee Replacement Devices Subject to Recall
Latest Article
What Is Second-Degree Murder?
How Does It Differ From Other Types of Homicide? In all states, homicide crimes include a range of offenses that can be... Read More
Is Annulment the Same Thing as Divorce?
What Is an Annulment? When Can a Marriage Be Annulled? Why Seek an Annulment Rather Than a Divorce? Your marriage isn't... Read More
What Is a Right-to-Work State?
What Protections Does a Worker Have in a Right-to-Work State? Is It the Same as Employment-at-Will? Have you ever heard... Read More
GETLEGAL®ATTORNEY DIRECTORY
Find Leading Attorneys in Your Area
NEED PROFESSIONAL HELP?
Talk to an Attorney
How It Works
- Briefly tell us about your case
- Provide your contact information
- Choose attorneys to contact you